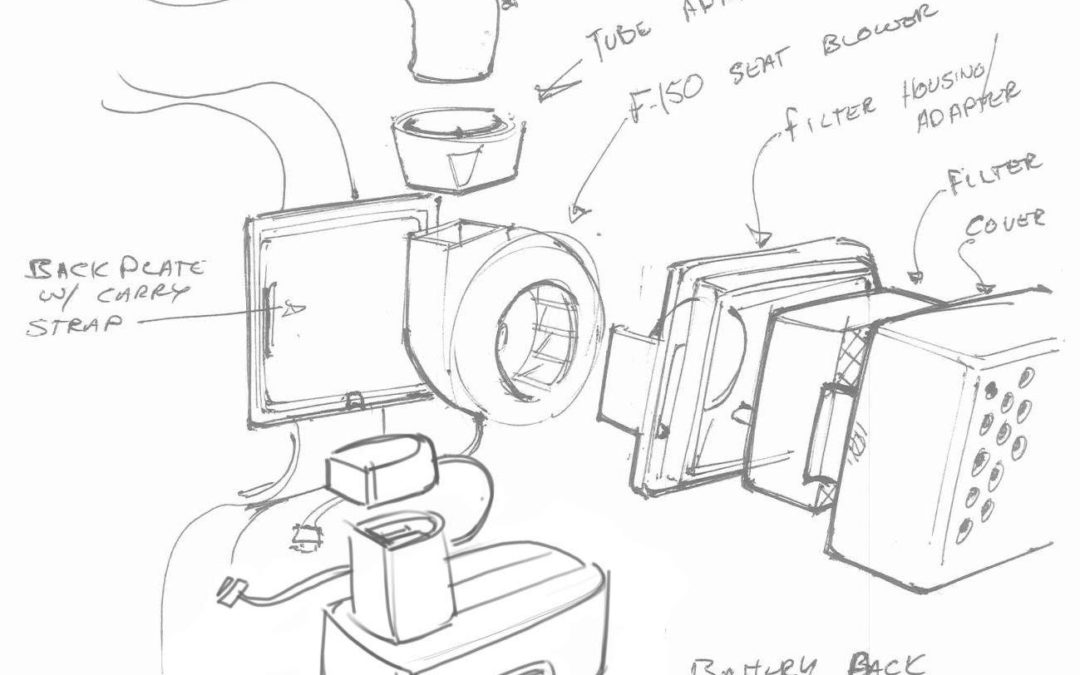
Can fans used to keep the seats of a pick up truck cool be repurposed to create more efficient medical ventilators? Can portable auto battery packs result in longer-lasting mobile medical equipment?
In a joint briefing this morning (March 24), Ford, GE, and 3M announced that they’re combining resources and expertise to respond to a dire need for ventilators, respirators, and medical protective equipment as coronavirus spreads across the US. The goal of the partnership is to try make the products most needed at this time easier to manufacture and more efficient.
Part of the initiative involves rethinking the design of 3M’s Powered Air-Purifying Respirators, a protective device responders use when attending to a sick patient.
“All this has inspired us to get scrappy,” says Jim Baumbick, Ford’s vice president of enterprise product line management. “How do we get creative and think about the basic design of the powered air-purifying respirator…in its ideal state and looking at the set of building materials and components that we already have?” The upgraded respirators will be produced by trained auto manufacturers in a Ford facility in Michigan.
In the face of a global economic shutdown, the three companies are part of a growing number of corporations who are reorienting their resources and supply chains to help arrest the coronavirus pandemic. LVMH’s perfume factories are making hand sanitizer while distilleries like BrewDog and 10th Mountain Whiskey & Spirit Company in Colorado are making anti-bacterial gels. And in the UK, the government is turning to the engineers of Rolls Royce and Dyson to also potentially help make ventilators.
“Ford has electronic components—blower units, fans, and components that can augment our supply chain for our existing product,” explains 3M CEO Mike Roman. “With a modified design, we are hoping to integrate other components, electronics, and injection modeling capabilities.” Beyond retooling the equipment, Ford and 3M are also aligning their production lines so all hoses, screws and the range of interchangeable industrial widgets are compatible.
“The goal being here is to increase volume very quickly,” explains Baumbick. “Time really here is the enemy.”
Apart from the respirators, Ford is also working with GE Health to simplify the design of its standard $50,000 bedside ventilator, customizing it for the novel coronavirus. As the infection rates grow by the day, US health experts say that the current stock of about 172,700 units won’t nearly be enough.
Though one might question the wisdom of going back to the drawing board during an emergency, the companies believe the new and improved medical equipment will create essential efficiencies in factory production and functionality. Baumbick says that Ford expects its efforts to result in a tenfold increase in the production of critical medical equipment for the Covid-19 pandemic.
Tom Westrick, vice president and chief quality officer for GE Healthcare, says they’re working with regulators from the Food and Drug Administration and National Institute for Occupational Safety and Health to fast-track the approval for the new designs. He didn’t have a timeline for when the simplified ventilators can be deployed, but says they’re “of utmost importance.”
Westrick adds that GE has provided information to clinicians on how to repurpose anesthesia machines for coronavirus response. “Anesthesia systems have ventilating capabilities that also can be used to meet Covid-19 patient [needs],” he explains. “We have a global installed base of over 100,000 of these devices. These devices can provide immediate assistance in the global demand for ventilators across the world.”
Ford is also deploying its fleet of 3D printers to manufacture 100,000 plastic face shields a week to augment the current supply of protective equipment.
This content was originally published here.